Thermogalvanic (TG) cells refer to electrochemical cells that convert heat into electricity in a device configuration similar to that of thermoelectric (TE) devices. It is well-known that the potentials of electrochemical reactions have temperature coefficients (often called thermogalvanic coefficient a) on the order of 1 mV/K, much higher than that of typical TE materials. However, the electrical conductivity of electrolytes is low, and thus the achieved efficiencies have been less than 0.5%. An alternative approach of electrochemical system for thermal energy harvesting is to explore thermodynamic cycle called thermally regenerated cycles (TREC): discharging the battery at a lower temperature and charging back at a higher temperature. If the charging voltage at the higher temperature is lower than the discharging voltage at the lower temperature, net energy is produced by the voltage difference, originating from heat absorbed at the higher temperature.
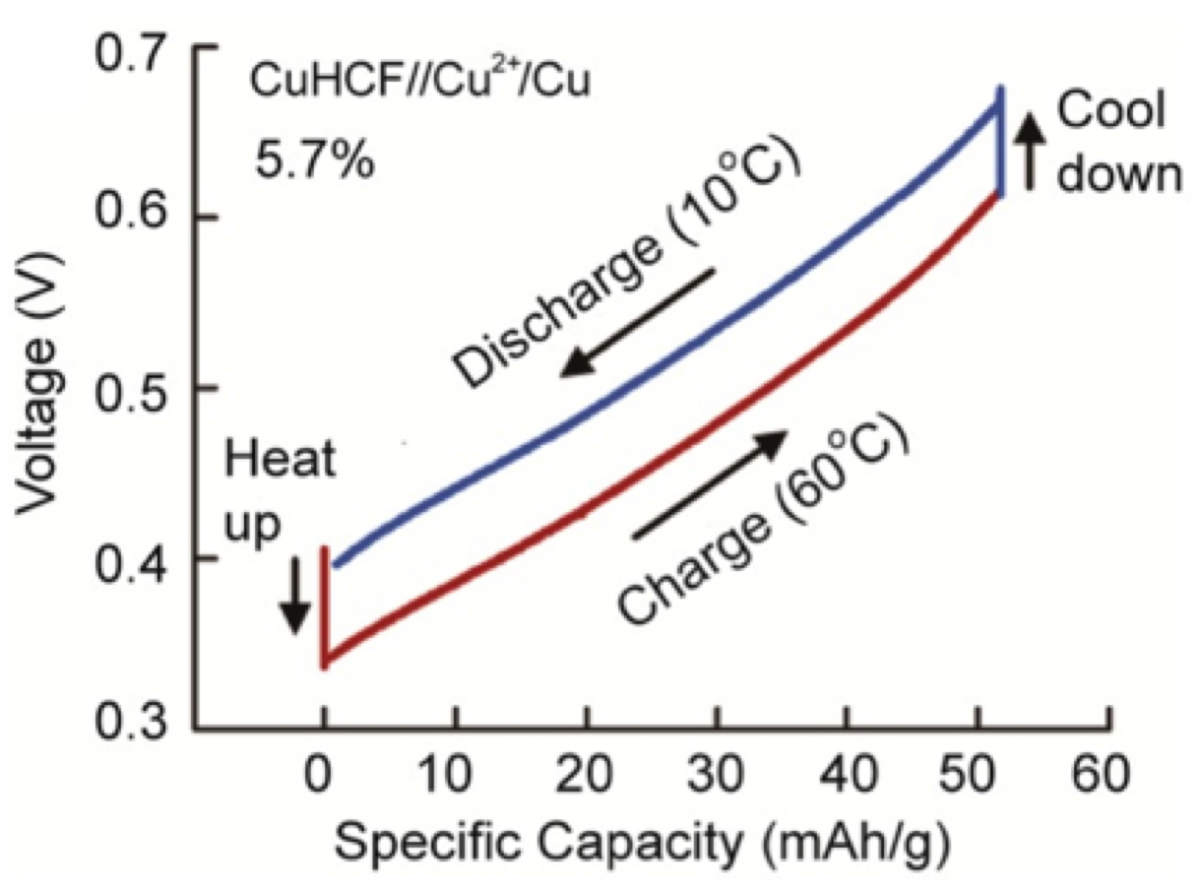
The concept of TREC was developed a few decades ago, but materials reliability and reversibility, and power density have limited their application. In collaboration with Prof. Yi Cui from Stanford, we invented a new TREC and achieved an experimental efficiency of 5.7% for cycles operating between Tcold=10oC and Thot=60 oC, based on a demonstrated heat recuperation efficiency of 50%, demonstrating the significant potential of TREC for recovering low-grade waste heat and converting it into electricity [1]. Subsequently we developed a charging-free secondary battery system based on Iron(II,III) hexacyanoferrate(II,III) (Prussian blue) and Fe(CN)63-/4- redox pair for TREC [2]. After discharging at 20 ºC, the battery is heated to 60 ºC and the spontaneous reaction direction (discharge) changes and becomes opposite to that at 20 ºC due to the temperature effect. Therefore the electrochemical process at 60 ºC is also discharge. Such a battery always discharges and no electrical charging is needed. Furthermore, We developed a membrane-free battery system for TREC to convert heat to electricity. The electrode materials are nickel hexacynoferrate (K1+xNiFe(CN)6) for the cathode and Ag/AgCl for the anode. Ions involved in the two electrode reactions do not interfere with the other electrode so that an ion-selective membrane is not needed to block certain ions. Therefore expensive ion-selective membranes can be removed from the system to lower the cost [3]. Our current research on TREC focuses on searching for better materials with larger electrochemical temperature coefficient, as well as understanding the fundamental connection between material entropy and the electrochemical temperature coefficient.
References:
1. S. W. Lee et al, An electrochemical system for efficiently harvesting low-grade heat energy, Nature Communications, 5, 3942 (2014)
2. Y. Yang et al, Charging-free Electrochemical System for Harvesting Low-grade Thermal Energy, PNAS, 111, 17011 (2014)
3. Y. Yang et al, A Membrane-free Battery for Harvesting Low-grate Thermal Energy, Nano Letters, 14, 6578 (2014)

